Technology Solutions for Decentralized and Hybrid Clinical Trials
Integrated Industry Leading Systems to Meet Your Trial’s Needs
SDC delivers comprehensive technology infrastructure tailored for decentralized, hybrid, and traditional clinical trials. From real-time eSource data capture to centralized analytics, our integrated platforms enhance trial compliance, efficiency, and patient engagement.
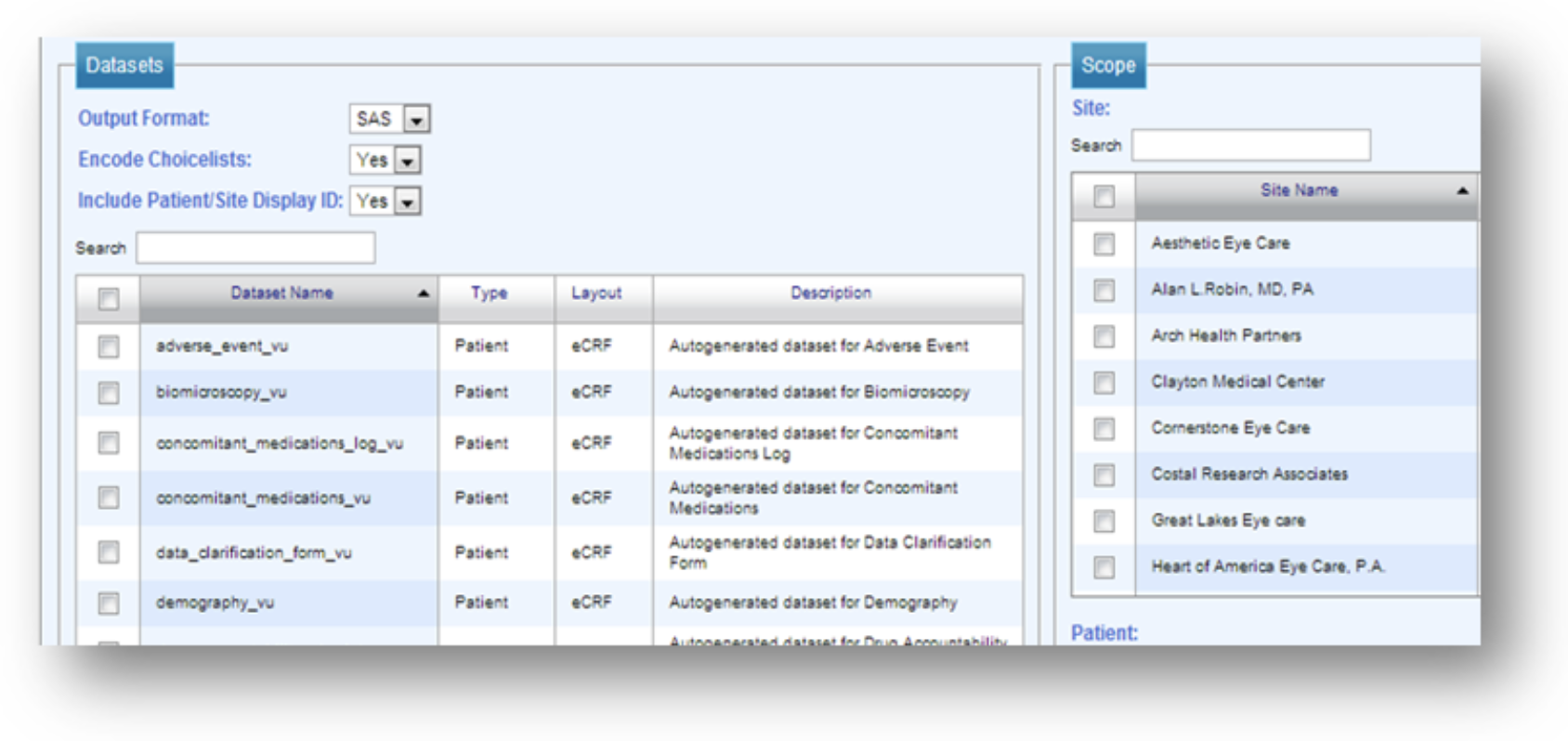
Our validated platforms for EDC, IRT, eSource, ePRO/eCOA, eConsent, and eRegulatory ensure seamless integration and compliance across all phases of your trial. Powered by SDC Insights, we centralize reporting across systems while reducing redundant data entry with our AI-enabled Data Hub.
With single sources of truth we ensure that the information generation comes from the correct source as reports are only as good as the data behind them.

SDC Capture™
SDC Capture provides an efficient and easy to deploy ePRO/eCOA solution to meet your cost and timelines needs.
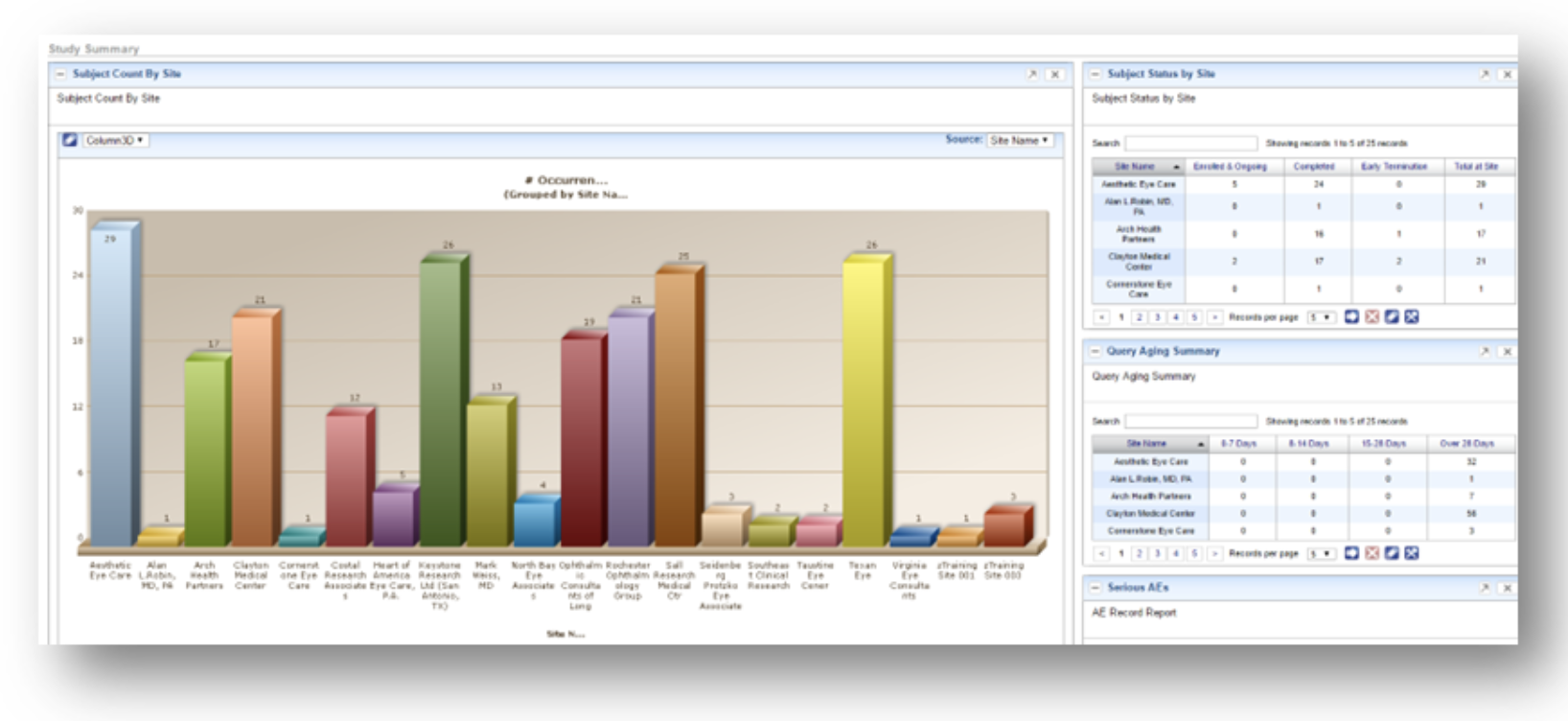
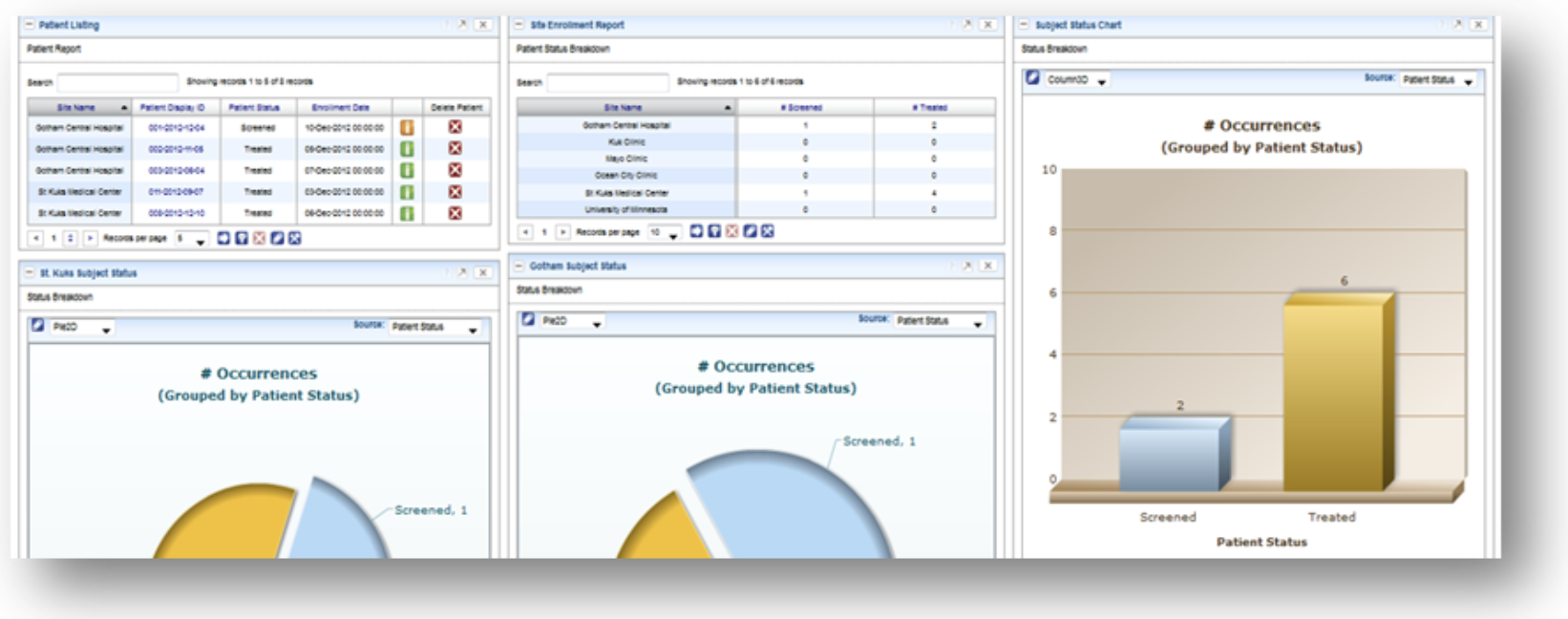
EDC / IRT
Our EDC and IRT solutions are built through trusted partnerships with industry leaders including Medidata, Oracle, and Mednet. Each platform is independently validated by SDC and configured based on study design, therapeutic area, and compliance requirements.
eSource
Through our partnership with CRIO, SDC offers real-time eSource solutions that eliminate paper, enhance data quality, and reduce site workload. Study staff can securely capture, monitor, and verify data with full audit traceability and compliance.
Safety / PVG
SDC provides integrated SAE collection within validated EDC systems (iMednet and Rave) as well as comprehensive global pharmacovigilance via SafetyEasy®. Ensure compliant, efficient safety reporting with real-time alerts, SUSAR tracking, and automated regulatory output.
Technology Solutions
Clinical Trial Budget Management
(CTBM)
Clinical Trial Management System
(CTMS)
Electronic Consent
(eConsent)
Electronic Clinical Outcomes Assessments
(eCOAs, ePROs, ClinROs)
Electronic Data Capture
Electronic Regulatory
(eRegulatory)
Electronic Source
(eSource)
Electronic Trial Master Files (eTMF)
Interactive Response Technology (IRT) for Randomization & Trial Supply Management (RTSM)
Investigator Portal
Patient Recruitment
Risk and Remote Based Monitoring
(RBM)
Safety & Pharmacovigilance
SDC Capture’s BYOD-enabled ePRO (electronic patient-reported outcomes) platform eliminates paper-based workflows, increases patient adherence, and streamlines clinical trial data collection. This 21 CFR Part 11-compliant system supports both bring-your-own-device (BYOD) and provisioned device strategies—maximizing accessibility and trial flexibility.
Key benefits of our BYOD ePRO solution include:
Eliminate Paper-Based Processes
SDC Capture fully digitizes patient-reported outcomes, eliminating paper forms to reduce errors, streamline clinical data management, and accelerate study timelines.
Lower Trial Costs with BYOD
By enabling participants to use their own devices, our BYOD ePRO solution minimizes hardware costs and simplifies logistics—making SDC Capture one of the most cost-effective ePRO platforms on the market.
Boost Patient Engagement and Adherence
The user-friendly interface integrates seamlessly into participants’ daily routines, enhancing protocol compliance, reducing patient burden, and improving both recruitment and retention.
Seamless Integration Across Devices
Our platform supports real-time data capture on both personal and provisioned devices, delivering a smooth experience across diverse participant populations and increasing data consistency.
Improve Clinical Data Quality
Real-time alerts, feedback, and support delivered directly to participants’ devices improve the accuracy and completeness of collected data—leading to better outcomes for researchers and patients.
SDC Capture is also 21 CFR Part 11 compliant and fully validated in alignment with the highest of industry standards.
EDC / IRT
Helping you make informed decisions.
SDC offers our clients leading technology options licensed via trusted and proven partnerships with the industry’s best providers. We evaluate each project based on study design, therapeutic area, location/language requirements, etc, and make recommendations to our clients regarding the optimal technology solution for their studies.
Our EDC and IRT solutions are built through trusted partnerships with industry leaders including Medidata, Oracle, and Mednet. Each platform is independently validated by SDC and configured based on study design, therapeutic area, and compliance requirements.
Mednet

Mednet is a healthcare technology company specializing in eClinical solutions designed for the global life sciences community. Mednet’s all-in-one eClinical platform improves the efficiency of clinical studies of all types and sizes. Beyond electronic data capture (EDC), Mednet’s comprehensive solution set provides the tools required to build and manage all types of clinical research, while enabling organizations to adapt to evolving demands and requirements.
Partnership Highlights:
SDC is the premier iMednet eClinical partner
SDC preferred EDC solution since 2012
200+ successful iMednet database builds, including 10+ successful product submissions/approvals
All EDC development services performed by SDC team
Cloud-based Software-as-a-Service Technology
Browser-based access; no software needed
Supports industry standards: CDISC, HIPAA, 21 CFR 11, GCP
Fully integrated IRT/ RTSM functionality
All EDC development services performed by SDC team
Full independent “fit-for-use” validation completed and maintained by SDC
Full integration with external systems via secure API including SDC Insights
Medidata

SDC has been supporting studies utilizing Medidata Rave for 10+ years. This experience has included 40+ studies across multiple therapeutic areas and all study phases.
Industry leading eClinical platform for 10+ years
SDC has been an accredited Medidata Build Partner since 2017
In-house custom function development and core configuration management
40+ Rave EDC builds completed, 20+ RTSM builds completed
Full integration with external systems via secure API including SDC Insights
Oracle Clinical One

Clinical One is a leading-edge platform that truly unifies people, processes, and data to simplify and accelerate the clinical trials of today and in the future. SDC has years of experience working with Clinical One to deploy large scale clinical trials.
Other Technology Options
In addition to these technologies, SDC has extensive experience and frequently supports studies in a variety of other eClinical platforms upon client request.
eSource

Through our partnership with CRIO, SDC offers real-time eSource solutions that eliminate paper, enhance data quality, and reduce site workload. Study staff can securely capture, monitor, and verify data with full audit traceability and compliance. SDC’s eSource system is a fully validated, 21 CFR Part 11-compliant platform designed for real-time data capture, increased efficiency, and enhanced data quality.
Core Benefits:
Significant Time Savings: Streamline data collection, reduce site burden, and minimize manual transcription tasks.
Improved Data Accuracy: Capture clinical trial data at the point of care, reducing errors and enhancing source data integrity.
Recruitment & Compliance Advantage: Improve recruitment quality while ensuring protocol and regulatory compliance.
Site-Level Flexibility: Customize forms and workflows for each protocol while maintaining consistency and audit readiness.
Advanced eSource Capabilities:
Real-Time Data Entry: Eliminate paper, reduce duplicate entry, and optimize data management workflows.
Remote Access & Oversight: Study staff can log in anytime to review the audit trail, queries, and document changes—without exposing CRA-facing data.
Document Workflow Automation: Upload and route lab reports, ECGs, and patient diaries; sign documents digitally and receive real-time PI signature alerts.
Reduced Monitoring Burden: Accelerate source verification and minimize site visits through live access to verified data.
Safety / Pharmacovigilance (PVG)
SDC delivers robust, flexible pharmacovigilance solutions for clinical trials—supporting streamlined SAE data collection, reporting, and regulatory compliance. Whether global surveillance is required or not, we offer validated, integrated safety workflows tailored to your trial needs.
Integrated eSAE Workflow within EDC
As the first CRO to deliver embedded SAE reporting directly within industry-leading EDC platforms, SDC offers a modern alternative to traditional safety data collection methods.
For trials not requiring global pharmacovigilance services, our eSAE solution offers:
End-to-End Safety Data Collection: Capture and report SAE, UADE, and AESI events directly from iMedNet or Rave.
Reduced Site Burden: Automatically pull adverse event data already entered into AE case forms—minimizing duplicate data entry
Validated EDC Integration: Enable SUSAR alerts, real-time processing, and compliance tracking within our fully validated platforms.
Real-Time Team Notifications: Automatically notify designated safety leads to ensure rapid response and documentation.
SafetyEasy®
For clinical trials requiring comprehensive global safety reporting and surveillance, SDC offers SafetyEasy®—a proven Multivigilance management suite used across drug, device, and cosmetic studies.
SafetyEasy® supports full compliance with FDA, EMA, and MHRA requirements and adapts to evolving global pharmacovigilance regulations. With an intuitive user interface, the platform streamlines adverse event data capture, coding, and regulatory submissions.
Key capabilities of SafetyEasy® include: