Safety / Pharmacovigilance
SDC recognizes the importance of robust yet flexible solutions when it comes to SAE data collection and reporting, and as such we now offer multiple integrated options to meet your safety/PVG needs.
eSAE workflow within EDC
SDC is proud to be the first CRO to offer our clients a unique alternative to traditional SAE data collection and reporting.
For studies not requiring global safety reporting or surveillance, SDC offers an eSAE solution:
Allows for end-to-end safety data collection and reporting directly from our preferred EDC platforms.
Reduces sites’ time burden while also pulling in data already entered into the AE case form.
Integrated Serious Adverse Event, UADEs, and AESIs data collection, processing, reporting and SUSAR reporting window alerts within our validated EDC systems, iMedNet and Rave, reducing manual workload while increasing accuracy and efficiency of data entry, collection and reporting.
Real-time notifications to specified study safety team members.
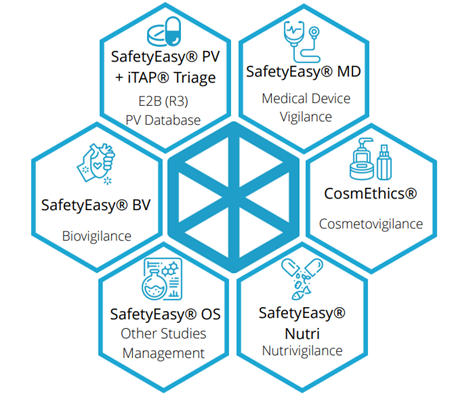
SafetyEasy®
For clients/programs requiring the gold-standard in global safety reporting and surveillance, SDC also offers Multivigilance management through the SafetyEasy® Suite. SafetyEasy® conforms to FDA, EMA, and MHRA requirements, while staying current with the everchanging regulatory landscape. SafetyEasy® provides an intuitive user interface for collecting and importing data, easily generating narratives, smart MedDRA coding, and one-click submission of regulatory reports in multiple formats (MedWatch, CIOMS, MedDev, etc.). SafetyEasy® helps manage adverse event reporting and stay compliant with current regulations and has been used for drug, device and cosmetic trials.

See why SDC is the Right Fit For You.
Contact us or email info@sdcclinical.com
Safety / Pharmacovigilance Case Studies
SDC Optimizes Focus on Tech-Enabled Biometric Services
Phoenix, AZ – Statistics & Data Corporation (SDC), a global leader in life sciences biometrics services and technology solutions, announces a strategic decision to enhance focus and efficiency...
Faith Haines Kolb Appointed President and Chief Operating Officer of SDC
Tempe, AZ – Statistics & Data Corporation (SDC), a leading provider of innovative clinical data solutions, proudly announces the promotion of Faith Haines Kolb to President and Chief Operating...
SDC Partners with Singular to Advance Secure AI Solutions in Healthcare
The collaboration introduces innovative AI technology while ensuring data security and the data you want and actionable insights you need at your fingertips. Tempe, AZ – SDC has partnered with...
