SDC Sidekick™
AI Clinical Trial Oversight That Thinks Ahead
Smarter insights. Fewer site visits. Zero surprises.
SDC Insights™ 2.0 featuring SDC Sidekick™ brings real-time clarity to clinical trials with centralized monitoring by unifying your oversight data—and amplifying it with SDC Sidekick™, our embedded explainable AI engine.
From predictive risk alerts to audit-ready automation, SDC Sidekick™ acts like an always-on, multi-specialist teammate. Whether you’re managing protocol complexity, responding to regulatory pressure, or working under resourcing constraints—Sidekick helps your team do more, earlier, with less.
Risk-Based Quality Management (RBQM) Made Simple
Transform your risk-based monitoring strategy with AI-powered oversight. SDC Insights™ 2.0 with SDC Sidekick™ AI enables centralized monitoring platform technology that reduces site visits by 23% while improving clinical trial risk detection speed and accuracy.
AI Clinical Trial Oversight – A New Generation Has Arrived
Today’s trials are anything but static. Data flows in constantly from decentralized sources—wearables, ePROs, CTMS, labs, EDC, and more. Manual review processes, siloed dashboards, and delayed KPIs can no longer keep up with the speed and scale of modern protocols.
That’s why we re-engineered SDC Insights as a fully connected centralized monitoring platform—and embedded SDC Sidekick AI to make it proactive, predictive, and explainable.
What’s New in SDC Insights 2.0
Here’s how SDC Insights 2.0 with SDC Sidekick AI brings visibility, efficiency, and regulatory confidence to every stakeholder across your trial operations:
|
Feature |
What It Means for You |
|
SDC Sidekick AI Integration |
Predict and prioritize risks faster with real-time AI models tuned for your specific study needs. |
|
Role-Based Dashboards |
Real-time clinical trial dashboards for CRAs, Safety Monitors, Biostats, and Clinical Ops—with no manual exports or Excel pivots. |
|
All-Source Harmonization |
Clinical data management AI seamlessly integrates EDC, CTMS, IRT, , lab, and wearable data—automatically structured and integrated in one secure platform. |
|
Explainable AI Outputs |
Transparent query generation, row-level traceability, and hot-key AI validation in GxP that meets/exceeds all regulatory requirements. |
|
FDA-Ready AI Oversight |
FDA-ready AI oversight tools with SOC 2 + ISO certification ensuring inspection readiness and regulatory confidence. |
|
Rapid Go-Live (≤30 Days) |
Live before First Patient In. No re-platforming. No middleware required. |
The Clinical Trial Reality: Why Sidekick Is Built for Now
Trials today are under pressure from every direction—compressed timelines, complex protocols, rising data volumes, and evolving regulatory expectations. SDC Sidekick AI clinical trial oversight is designed to meet these challenges head-on with measurable results.
Here’s what teams using SDC Sidekick™ are already seeing:
|
23% reduction in site-monitoring visits |
Based on clinical trial site monitoring automation in a live Phase II oncology study |
|
60% of queries auto-drafted |
AI for clinical data management saves 4+ hours per CRA, per week |
|
14 days earlier detection of risk trends |
Clinical trial anomaly detection AI stays ahead of DLTs, protocol deviations, and safety outliers |
|
100% mock audit pass rate |
Audit-ready clinical trial data with traceable, explainable AI outputs with built-in validation |
|
Deployment in <30 days |
Including pre-FPI environments |
|
All-source integration |
Clinical operations risk management works with any EDC platform—yours or third-party |
This is just a sampling of value and impact. There are many more ways to improve clinical research.
Empowering Every Oversight Role, Every Day
SDC Insights™ 2.0 featuring SDC Sidekick™ isn’t just a platform—it’s a purpose-built partner for every function that touches trial data. Whether you’re triaging queries, leading a CRA huddle, reviewing safety narratives, or managing global operations, SDC SidekickTM AI brings precision to your decisions.
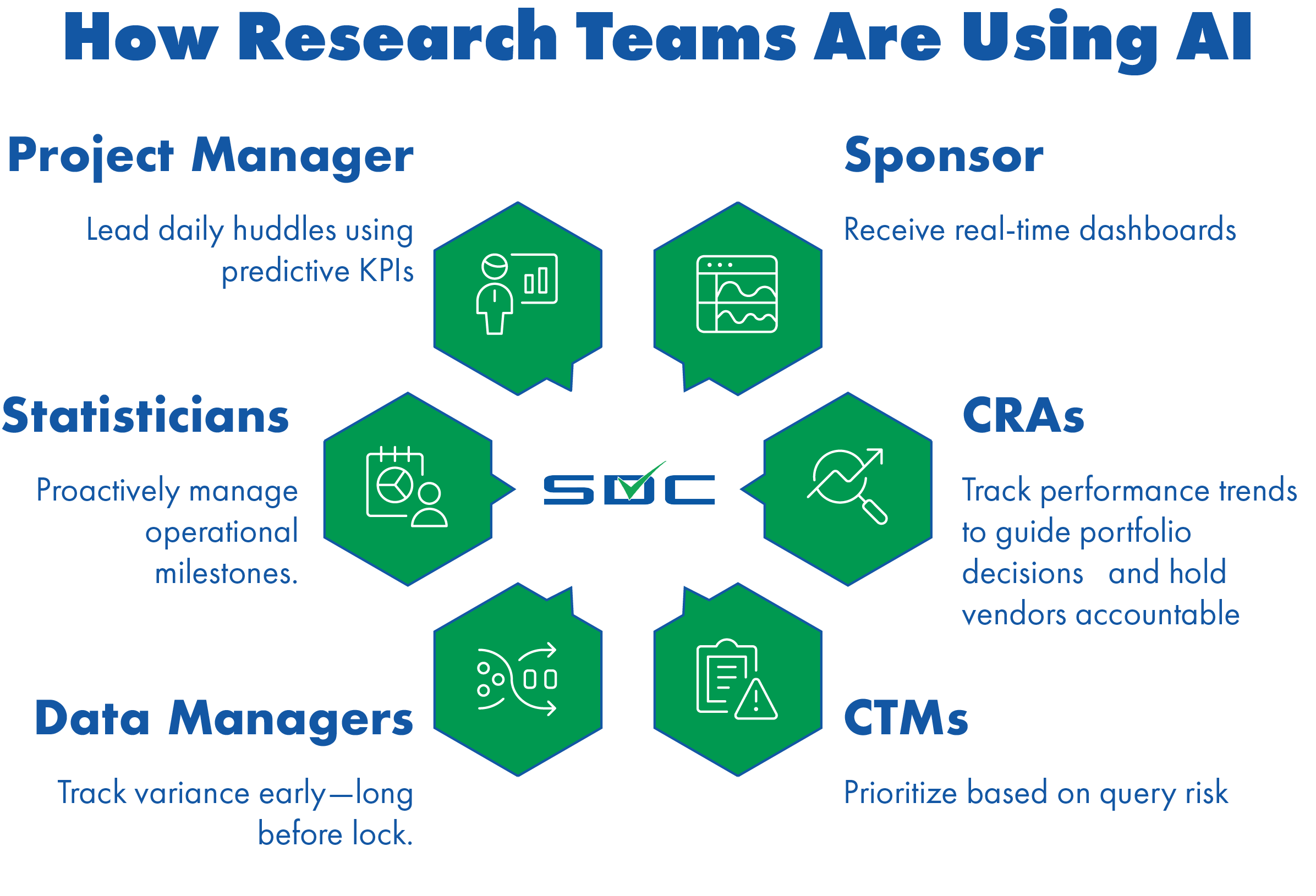
| Stakeholder | How SDC SidekickTM Adds Value |
| Clinical Ops | Monitor risk in real time, track CRA and site performance, and hold vendors accountable. |
| Biometrics & Data Management | Automate multi-source harmonization and focus effort where query risk is highest using clinical trial risk detection platform technology. |
| CRAs & CTMs | Cut huddle prep time by 40% and prioritize site actions with predictive clinical trial dashboards powered by AI clinical trial oversight. |
| Medical Monitors & Safety | Detect safety signal clusters across labs and EDC—before they escalate. |
| Sponsors & Procurement | Reduce SDV and oversight costs without adding FTEs—and without waiting until mid-study. |
FDA-Ready. Audit-Tested. ICH E6(R3) Compliant.
Oversight tools that rely on “black box” algorithms or manual data exports are not only outdated—they’re noncompliant.
SDC Sidekick™ is purpose-built to meet the highest regulatory standards for transparency, traceability, and validation:
- SOC 2 Type II
- ISO 27001
- 21 CFR Part 11
- Hot-key revalidation of all AI outputs
- Zero FDA 483s across live pilot studies
- Ready for ICH E6(R3) compliance solutions starting June 2026
If your audit trail doesn’t show what your AI saw, you’re not audit-ready. With SDC Sidekick™, you are.
Fast to Deploy. Easy to Scale. No Rebuilds Needed.
Most oversight systems take 60–90 days to implement—and even longer to show value. Sidekick was built differently.
- Go live in ≤ 30 days, including pre-FPI
- No re-platforming required—works seamlessly with your current EDC/CDMS
Whether you’re running one study or scaling oversight across a portfolio, SDC Sidekick™ evolves alongside your clinical programs.
Trusted by the Teams Driving Oversight Innovation
“We cut query triage time in half and finally have one dashboard that gives our team the full story—from safety signals to site performance.”
“Sidekick made us stand out in a recent RFP. The explainability and speed to deploy are unmatched.”
“SDC Insights™ identified restrictive I/E criteria that slowed enrollment and allowed us to quickly mitigate enrollment challenges and expedite our study timelines.”
Ready to Think Ahead?
You don’t need more dashboards.
You need a smarter way to see risk, act faster, and improve your process.
Mine data for any answer in real time, with the strength of a CDM, biostatistician, safety reviewer, and programmer right at your side.
Let SDC Sidekick™ do the heavy lifting.
